Don´t leave your User in the darkness
Rent a CLINICAL ADVISOR, USABILITY EXPERT and RISK MANAGER for MEDICAL DEVICES

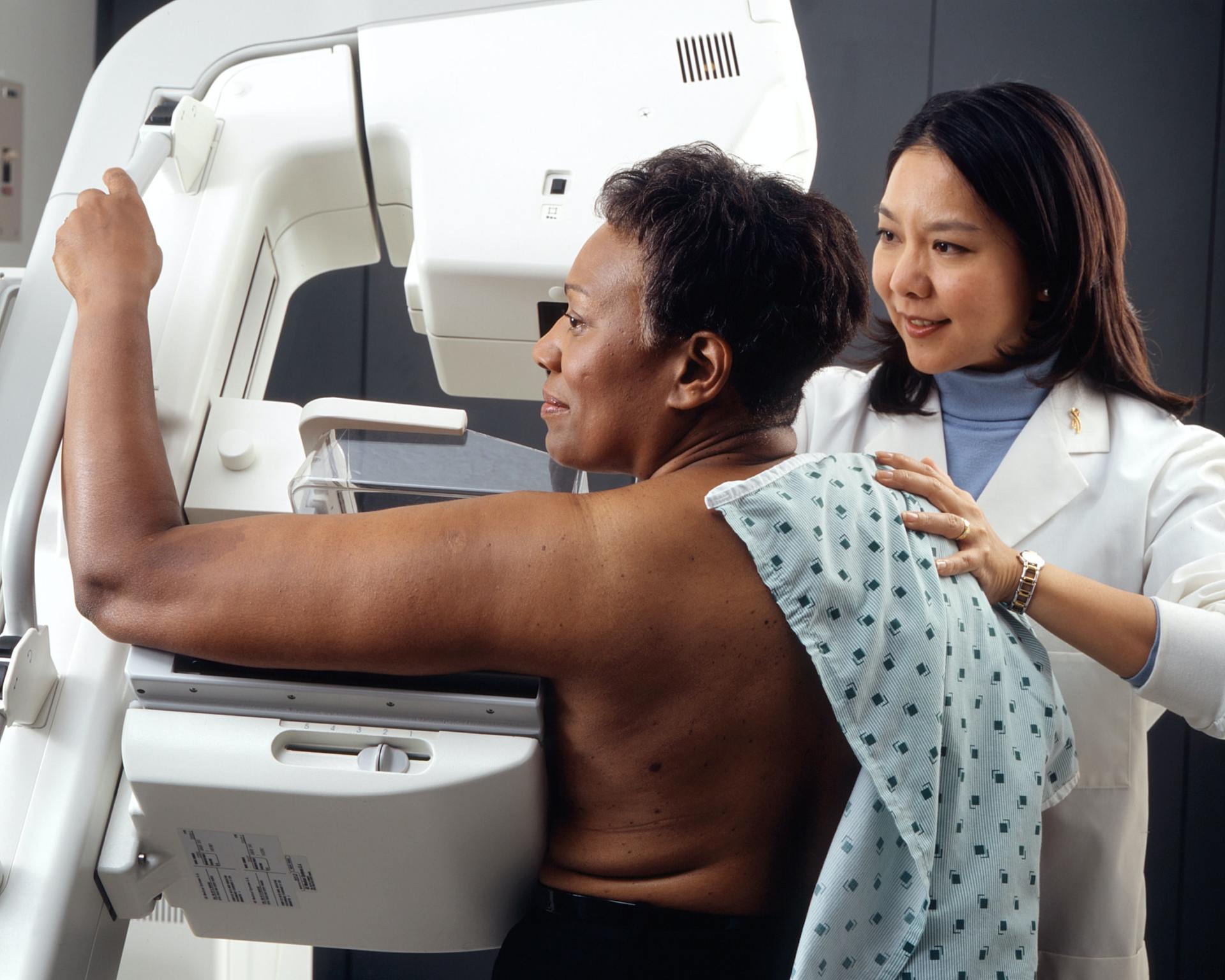
Usability Test
performing formative and summative usability testing in clinical or ambulance environment, incl. the recruitment of medical professionals according to the user profile.

providing a spectrum of services like:
- agile project management
- recruiting of medical staff depending on your user profiles
- programmable clinical simulation, including emergencies and troubleshooting
- recording of the usability test
- execution of formative and summative usability tests
- counseling by usability and work domain experts
- usability documentation according to EN ISO 62366 and FDA guideline
- risk management according to ISO 14971
- creating safety concept and alarms according to IEC 60601-1-8
- recommendations for user interface functions and design
- systematic analysis of the therapy standards relating to usability
- collection of clinical data relating to the use specification
- usability review of complaints
- medical review of complaints
- evaluation of medical devices with user interfaces of unknown provenance (UOUP)
- and more ...
What is to do?
According to Medical Devices Regulation 2017/745 (MDR) and other national regulations manufacturers of medical devices need to provide data to demonstrate the safety and effectivity of their devices. The requirements of MDR and the European Regulation 2017/746 on in vitro diagnostic medical devices (IVDR) ask for a lifecycle scrutiny by post-market surveillance (PMS) and post-market clinical follow-up (PMCF) reports. Manufacturers are challenged to design user interfaces that will not only satisfy regulatory requirements but will also perform safely and effectively under continued observation. This requires a user interface tailored to the user requirements, proper documentation of the usability design process, usability testing with intended users and usability risk analysis.
Risk Management ISO 14971
- the definition of acceptance criteria for risk analysis
- the identification of hazards from medical devices
- risk assessment and control
- the consultation on and control of risk management plans
- the control of verification and validation reports
- the consultation on and control of Risk Management Reports
- review of Clinical Evaluation Reports
Please, simply contact for more information
Thank you very much for your interest. We will provide our feedback as soon as possible.
Your Medical Usability Unit
Sorry, we had a communication error. Please, try again.
Impressum
Angaben nach §5 Telemediengesetz (TMG)
Betreiber dieser Webseite und verantwortlich
Ralf Thölke
Chausseestr. 118a
15712 Königs Wusterhausen
Tel. +4910608753102
E-Mail: ralf.thoelke@gmx.de
Steuernummer: 049/237/15113
USt-IdNr:: DE350157114
Alle Rechte vorbehalten | Ralf Thölke